Manage business operations with confidence on data that is governed and compliant
Master data is the heart of the data ecosystem.
A vision toward clean and reliable data
Establish state of the art master data management (MDM) services with reliability of operational data for business and compliance to
efficiently manage commercial operations and adhere to global mandate.
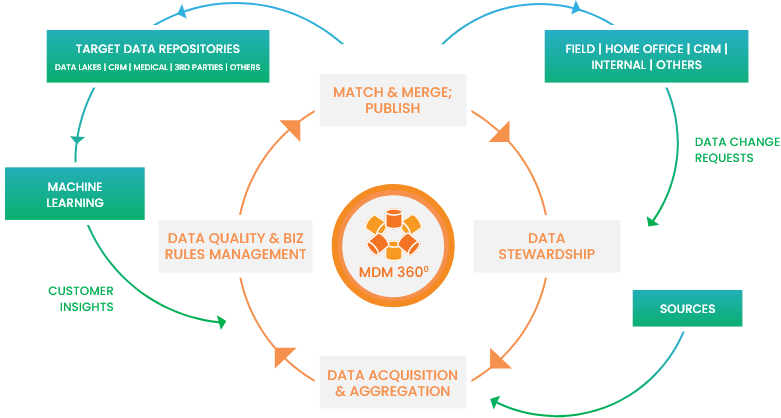
Bringing a single authoritative view of business-critical data
Enabled by a unique blend of people, domain knowledge, process, and technology
Strategy and consulting
Strategy and consulting
- MDM tool and roadmap assessment
- Data strategy and governance practices
- Process consulting
- DCR process
- New source onboarding
- Data enrichment, integration, and synchronization
MDM implementation
MDM implementation
- New MDM deployments
- Migration from custom processes or legacy MDM to cloud-based state-of-the-art application
- Data stewardship
- Upgradation and consolidation of existing applications
Entity domains
Entity domains
- Customer (HCO, HCP, and Affiliations)
- Individuals (patients and consumers)
- Payer/plan
- Product, Bill of Material (BOM), and equipment
- Vendor/supplier/distributors
- Clinical investigators/sites
MDM operations
MDM operations
- Ongoing support
- Vendor management
- DCR and Merge Queue resolution
- Stewardship services
- Operationalize data governance
Key benefits
Reliable operational data, on-demand
Multidomain, affiliations, and hierarchy management
Fast, CoE-driven solution deployment
Managed services – IT and infrastructure
Fast onboarding of structured and unstructured data sources leveraging ‘common format’
Automated and rule-based data stewardship

.png?width=1367&height=219&name=SalesIQ-differentiators-graphicBG%20(1).png)
Axtria research hub
Customer success stories

Case Study
Rebuilding The Customer Master Data System To Enhance Business Process And Realize Maximum Benefits

Case Study
Unlocking 33% Performance Gains With Commercial Data Management For Top Pharma

Case Study